Find the ratio of the masses m1 m2 – Embark on a fascinating journey into the realm of mass ratio, where we uncover the intricacies of comparing the masses of two objects. From everyday applications to scientific endeavors, the concept of mass ratio plays a pivotal role in our understanding of the physical world.
Dive in and discover the methods, applications, and factors that shape the enigmatic world of mass ratio.
Mass ratio, expressed as m1/m2, provides a quantitative measure of the relative heaviness of two objects. Its applications span a wide spectrum of disciplines, from chemistry and physics to engineering and manufacturing. Whether you’re balancing chemical equations, designing aircraft, or simply scaling a recipe, understanding mass ratio is essential.
Understanding the Concept of Mass Ratio
Mass ratio, denoted as m1:m2, represents the relative amounts of two different masses (m1 and m2). It provides a quantitative comparison of their magnitudes without specifying their actual values. Understanding mass ratio is crucial in various scientific and everyday applications.For
instance, in chemistry, mass ratios are used to determine the proportions of elements in a compound. In cooking, recipes often specify the mass ratios of ingredients to ensure the correct balance of flavors and textures. In engineering, mass ratios are employed to design structures and vehicles that can withstand specific loads and forces.
Examples of Mass Ratio Calculations in Everyday Life
*
-*Baking a cake
A recipe may call for a mass ratio of flour to sugar of 2:1. This means that for every 2 grams of flour used, 1 gram of sugar is required.
-
-*Mixing paint
To create a specific shade of paint, a painter might mix two colors in a mass ratio of 3:1. This means that for every 3 grams of the first color, 1 gram of the second color is added.
-*Calculating the mass of a compound
If the mass ratio of hydrogen to oxygen in water is 1:8, and the mass of hydrogen is 2 grams, the mass of oxygen in the water can be calculated as 8 x 2 = 16 grams.
Methods for Determining Mass Ratio: Find The Ratio Of The Masses M1 M2
Determining the mass ratio of two objects is a fundamental task in many scientific and engineering applications. Mass ratio provides valuable information about the relative sizes and densities of objects, allowing for comparisons and calculations in various fields.
Using a Balance or Scale
One common method for measuring mass ratio is using a balance or scale. A balance or scale measures the weight of an object, which is directly proportional to its mass. By placing two objects on the balance and comparing their weights, we can determine their mass ratio.
Formula for Mass Ratio
The mass ratio of two objects, m1 and m2, is calculated using the formula:
Mass ratio = m1/m2
Where:
- m1 is the mass of the first object
- m2 is the mass of the second object
Steps to Calculate Mass Ratio
- Place the first object on the balance or scale and record its weight, W1.
- Remove the first object and place the second object on the balance or scale. Record its weight, W2.
- Calculate the mass ratio using the formula: Mass ratio = W1/W2.
Applications of Mass Ratio
Mass ratio, a fundamental concept in science and engineering, plays a crucial role in various fields, from chemistry and physics to engineering, manufacturing, and even everyday applications.
Chemistry
In chemistry, mass ratio is essential for determining the composition of compounds. By analyzing the mass ratio of different elements present in a compound, chemists can establish its empirical formula, which represents the simplest whole-number ratio of the constituent elements.
Physics
In physics, mass ratio is used to study the behavior of objects in motion. For instance, the mass ratio of a projectile and the medium through which it travels affects its trajectory and velocity.
Engineering and Manufacturing
In engineering and manufacturing, mass ratio is critical for designing and optimizing structures and components. Engineers consider the mass ratio of different materials to achieve desired properties, such as strength, durability, and efficiency.
Everyday Applications
In everyday life, mass ratio finds applications in cooking and recipe scaling. By adjusting the mass ratio of ingredients, cooks can modify the quantity of a recipe without altering its proportions.
Factors Affecting Mass Ratio
Mass ratio, as discussed earlier, is the ratio of the masses of two objects. Various factors can influence this ratio, primarily density, volume, and shape.
Density, Find the ratio of the masses m1 m2
Density is a measure of how tightly packed the mass of an object is within a given volume. Denser objects have more mass packed into the same volume compared to less dense objects. The mass ratio of two objects with different densities will be directly proportional to their densities.For
example, consider two objects of the same volume, one made of lead (density: 11.34 g/cm³) and the other made of aluminum (density: 2.70 g/cm³). The mass ratio of the lead object to the aluminum object will be 11.34 / 2.70 ≈ 4.2, indicating that the lead object has a mass approximately 4.2 times greater than the aluminum object.
Volume
Volume is the amount of three-dimensional space occupied by an object. Objects with larger volumes have more space for mass to occupy compared to objects with smaller volumes. The mass ratio of two objects with different volumes will be directly proportional to their volumes.For
instance, consider two objects of the same density, one with a volume of 10 cm³ and the other with a volume of 5 cm³. The mass ratio of the larger object to the smaller object will be 10 / 5 = 2, indicating that the larger object has a mass twice that of the smaller object.
Shape
Shape can also affect the mass ratio of two objects, even if they have the same density and volume. This is because the distribution of mass within an object can vary depending on its shape.For example, consider two objects with the same density and volume, one shaped like a sphere and the other like a cube.
The mass ratio of the sphere to the cube will be slightly less than 1 because the sphere has a more compact shape, resulting in a more efficient packing of mass.
Visualizing Mass Ratio Data
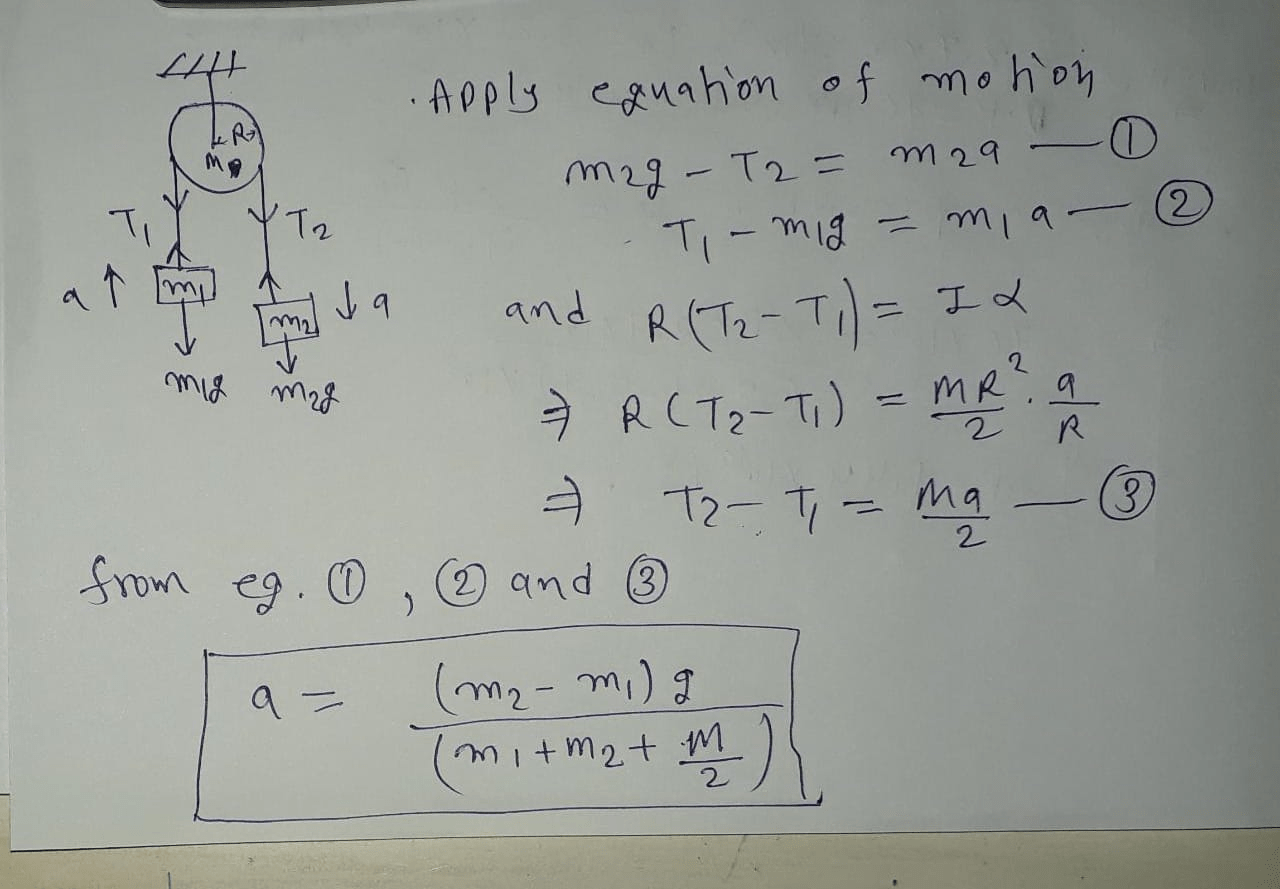
To effectively understand and communicate mass ratio information, visual aids play a crucial role. These aids help organize, present, and illustrate data, making it easier to identify patterns, relationships, and insights.
One effective method for organizing mass ratio data is through the use of a table. A table provides a structured format to list and compare mass ratios, allowing for quick and easy comparisons between different samples or variables.
Creating a Table to Organize Data
When creating a table to organize mass ratio data, it is important to include the following elements:
- Sample or Variable:Identify the samples or variables for which the mass ratios are being presented.
- Mass Ratio:List the calculated mass ratios for each sample or variable.
- Units:Specify the units in which the mass ratios are expressed (e.g., kg/kg, g/g).
Frequently Asked Questions
What is the formula for calculating mass ratio?
Mass ratio is calculated using the formula: m1/m2, where m1 and m2 represent the masses of the two objects being compared.
How is mass ratio used in chemistry?
In chemistry, mass ratio is crucial for balancing chemical equations and determining the stoichiometry of reactions.
What factors can affect mass ratio?
Factors that can affect mass ratio include density, volume, and shape.